APT introduces CO2 database for North Sea CCS players
28 January 2021 – Applied Petroleum Technology (APT) has compiled a large pressure-temperature-CO2 database for the North Sea to support operators that consider offshore storage of CO2.
The storage of CO2 in the subsurface has been proposed as a means to reduce CO2 release into the atmosphere, but this will require an understanding of the rock-fluid interactions. The introduction of CO2 into a reservoir system can lead to the dissolution of certain minerals that will alter the intrinsic physical and geomechanical properties of both the reservoir and seal, which in term can potentially reduce seal capacity (e.g. Kim & Santamarina, 2014). Alternatively, the introduction of CO2 in a reservoir can lead to mineral precipitation – securely sequestering the carbon. Whether mineral dissolution or precipitation occurs will depend on the reservoir pressure and temperature, mineral and formation water composition.
CO2 has three principal sources in the subsurface: from organic carbon buried in the sediment, from carbon stored within principally carbonate minerals and from the deep Earth. Upon burial sedimentary organic matter is converted first to a complex macro-molecule (‘kerogen’) during early diagenesis, which itself is then modified with progressive burial, undergoing thermal decarboxylation between ~50-100°C producing CO2. Carbonate minerals may form CO2 via dissociation due to chemical buffering with silicates (typically >70°C). Finally, CO2 may originate from deep-seated sources from either the thermal breakdown of carbonates or from volcanic sources. The latter source leads to the highest concentrations in sedimentary basins and can have important consequences for petroleum exploration in such basins, however such areas, with already high CO2 levels in the subsurface ,may be avoided as potential sites for carbon capture and storage (CCS). With respect to CO2 storage understanding the inorganic reactions between carbonates and silicates is of most importance, since potentially significant changes in physical properties of the rocks may occur as a result (Noiriel & Daval, 2017).
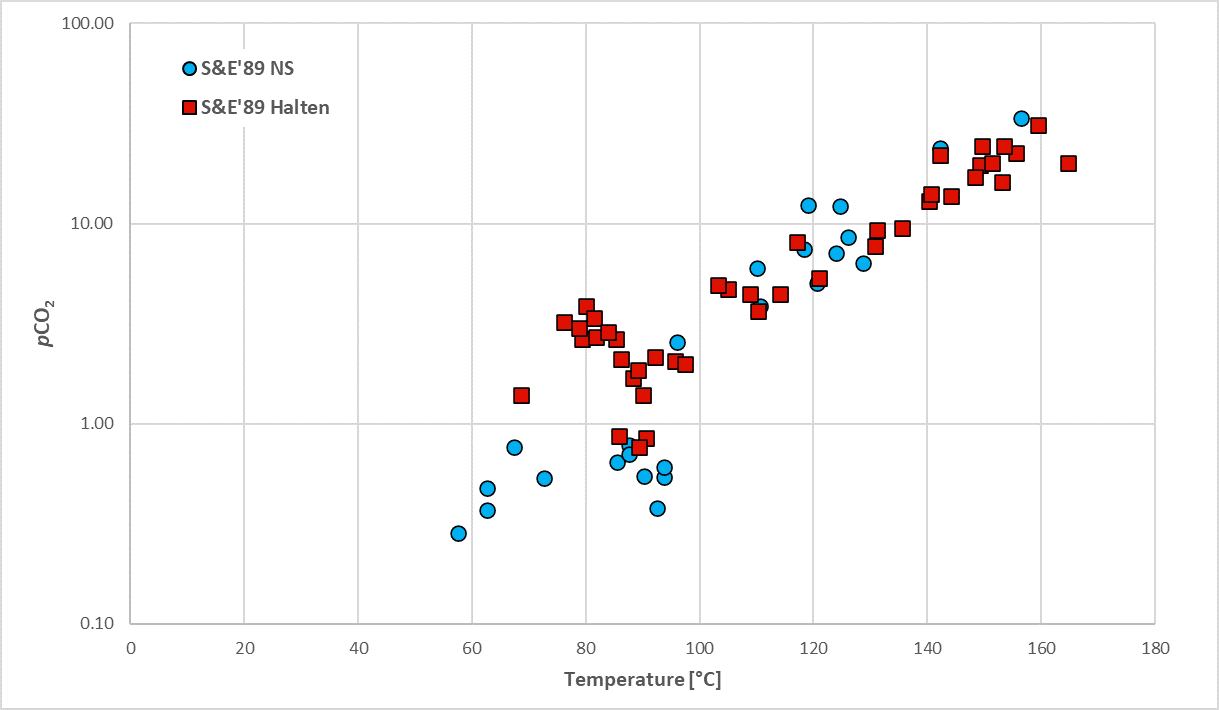
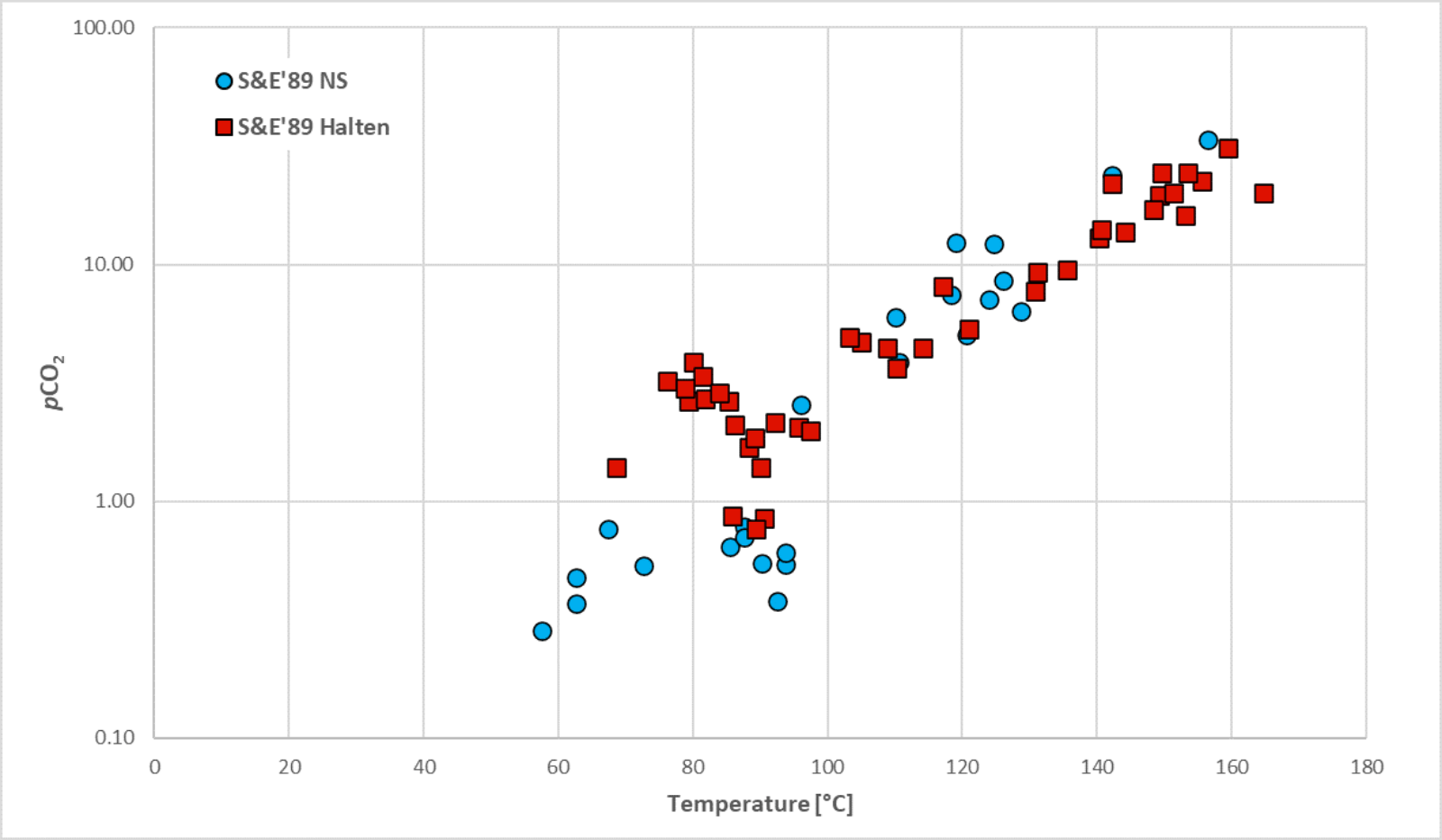
Figure 1. pCO2 versus temperature for petroleum reservoirs from the Norwegian North Sea and Haltenbanken basins (re-drawn from Smith & Ehrenberg, 1989).
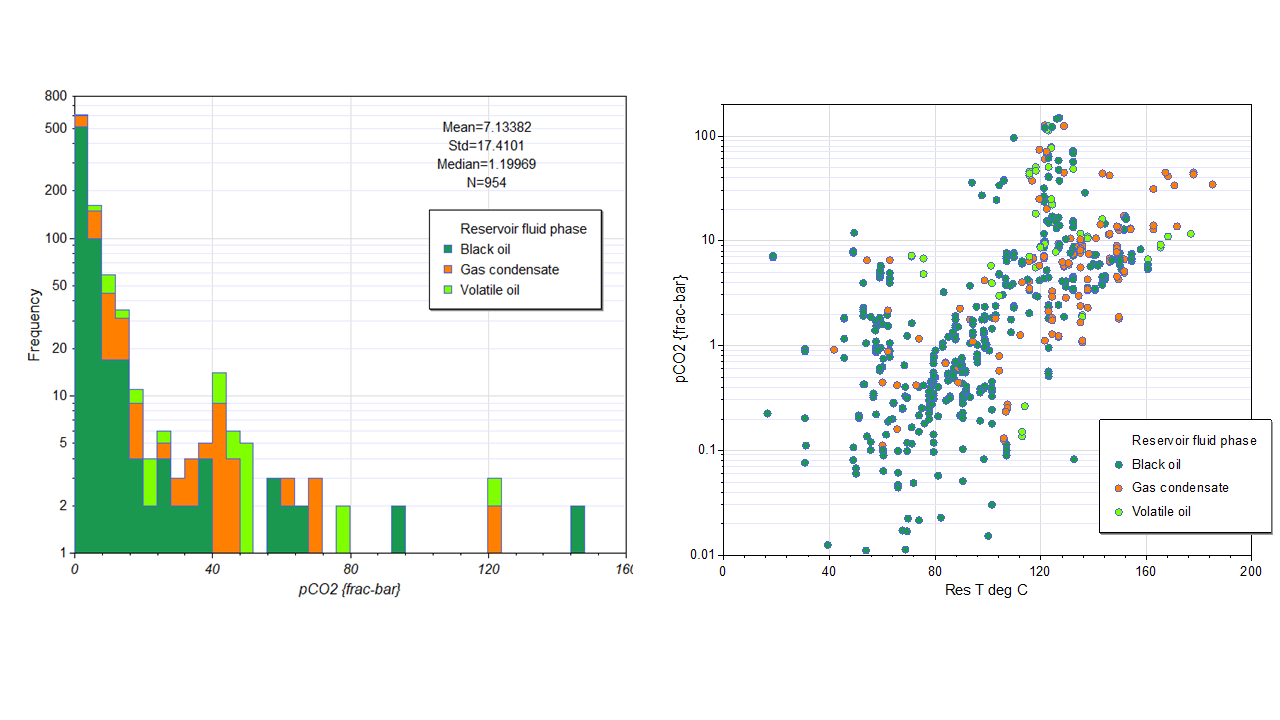
Figure 2. APT fluid CO2 database for petroleum fields UK sector North Sea.
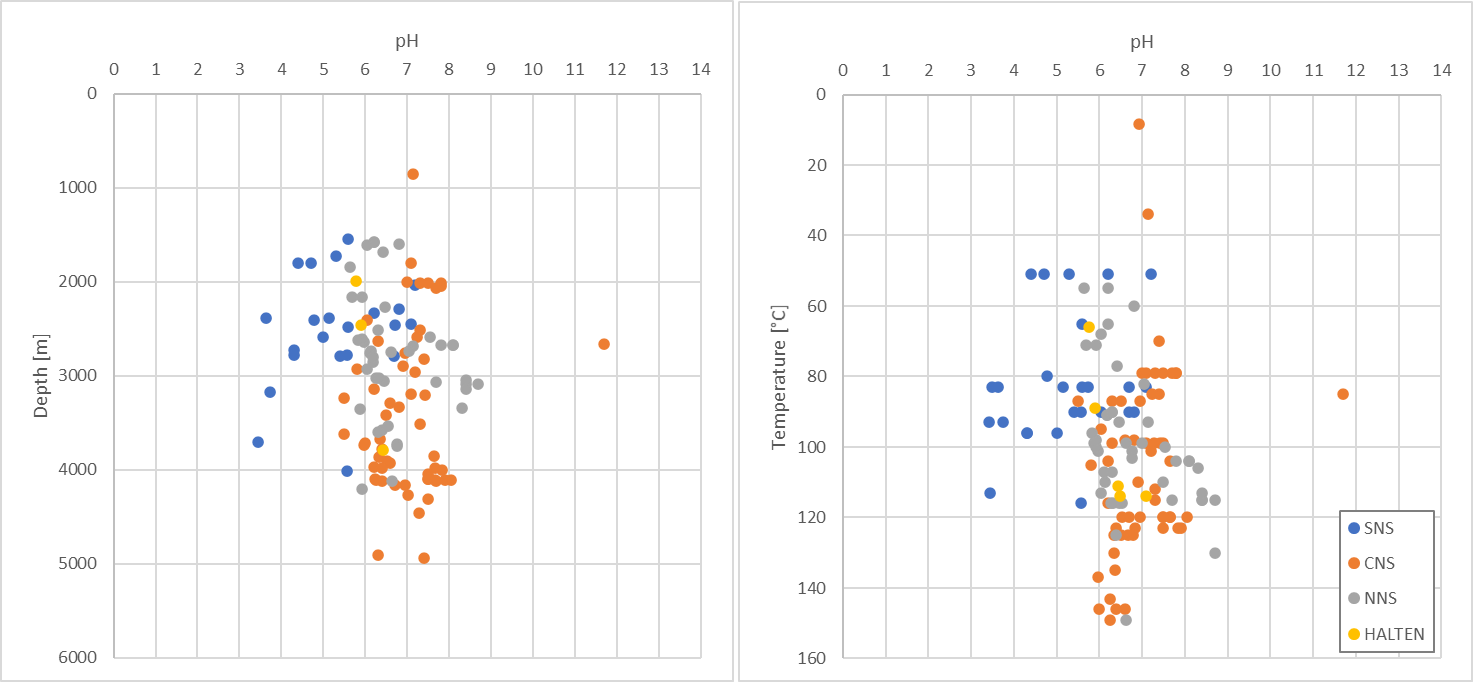
Figure 3. Formation water pH vs depth (m) and vs temperature (°C).
It is known that the CO2 concentration is controlled by buffering by feldspar, clay and carbonate minerals. CO2 and hydrogen ions (acidity) reach a temperature mediated equilibrium which is strongly dependent on the mineralogical composition. While the mineralogical make-up is typically either unknown or uncertain, plots of temperature vs the partial pressure of the CO2 (pCO2) exhibit clear trends in disparate clastic and volcanic basins, as shown in Figure 1 (cf. Smith & Ehrenberg, 1989; Hutcheon & Abercrombie, 1990). This indicates that all the potential reactions between feldspar, clay and carbonate minerals result in an equilibrium relationship between the partial pressure of the CO2 (mol% CO2 * fluid pressure, as an estimate of the fugacity) and temperature.
However, assessment of data can also play an important role in understanding what fluid-rock interactions are taking place. To that end, we have compiled a large pressure-temperature-CO2 database for the North Sea, as shown in Figure 2. The relationship between temperature and pCO2 is present but additional complexity is clearly also present and may indicate additional processes such as biodegradation or the recent addition of CO2 to the reservoir from a deep source (that has had time to equilibrate), furthermore we have observed some distinct local trends which may relate aH+ (where Formation waters are saturated with CO2). Plotting formation water pH demonstrates that water chemistries are generally buffered (Figure 3). We’re currently exploring what further insights can be drawn.
The database currently includes 954 pressure-temperature-CO2 mol % data points. This forms part of the APvT database (https://www.apt-int.com/services/apvt).
For information on accessing the data and for potential research collaboration please contact Julian Moore. Julian.moore@apt-int.com
References.
Hutcheon, I. and Abercrombie, H. 1990. Carbon dioxide in clastic rocks and silicate hydrolysis, Geology, 18, 541–544.
Kim, S. & Santamarina, J.C. 2014. CO2 geological storage: hydro-chemo-mechanical analyses and implications. Greenhouse Gas Science & Technology 4(4), p. 528–543.
Noiriel, C., & Daval, D. 2017. Pore-Scale Geochemical Reactivity Associated with CO2 Storage: New Frontiers at the Fluid−Solid Interface. Accounts of chemical research, 50, p. 759−768.
Smith, J. and Ehrenberg, S. 1989. Correlation of carbon dioxide abundance with temperature in clastic hydrocarbon reservoirs: relationship to inorganic chemical equilibrium. Marine and Petroleum Geology, 6, 129–135.